


- Stock: In Stock
- Model: 176170
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
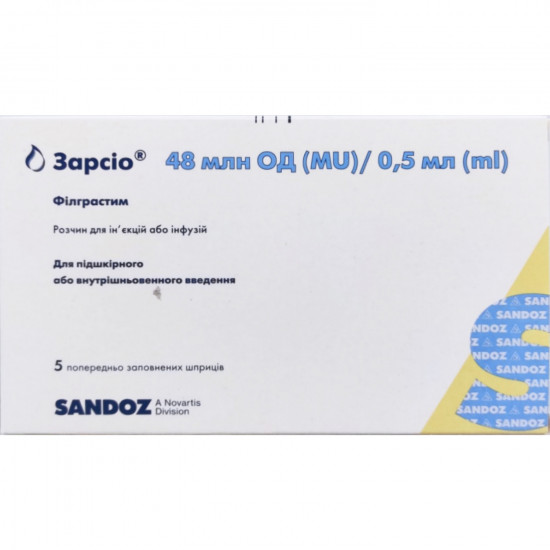
Reviews Over Zarsio solution for infection. and inf. 48 mln units / 0.5мл syringe No. 5 ***
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Solution for injections and Zarsio's infusions is shown for reduction of duration and weight of a neutropenia at the patients receiving intensive myelosuppressive chemotherapy cytotoxic medicines concerning malignant new growths (except for a myelosis and MDS) and reduction of duration of a neutropenia at patients who receive receiving high-dose chemotherapy cytotoxic medicines with the subsequent autologous or allogenic bone marrow transplantation.
Structure
Active ingredient: filgrasty (recombinant human granulotsitarny colony stimulating factor);
Previously filled the syringe (syringe dose) contains 48 mln units (480 mkg) of a filgrastim in 0.5 ml;
Excipients: glutaminic acid, sorbite (E 420), polysorbate 80, water for injections.
Contraindication
- Hypersensitivity to a filgrastim, colony stimulating factors, Escherichia coli or to any excipients.
- Heavy hereditary neutropenia (Kostman's syndrome) with cytogenetic violations and an autoimmune neutropenia.
- End-stage of the chronic kidney disease (CKD).
- Myelosis and miyelodisplastichesky syndrome.
Route of administration
Therapy of Zarsio® can be carried out byin medical institutions where there is a necessary diagnostic equipment. Doctors have to have experience of use of the medicines containing a granulotsitarny colony stimulating factor (G-KSF) and treatments of patients with hematologic diseases.
should be carried out byProcedure of mobilization and an aferez in interaction with doctors who have the corresponding experience and a possibility of necessary monitoring of progenitors of a hematopoiesis.
Drug to dissolve 5% of solution of glucose in 20 ml and to apply in the form of short infusion within 30 minutes of long hypodermic or intravenous infusion within 24 hours.
Pregnant
it is not recommended by
Feature of application
to p to application. ChildrenAt application in children's practice by the patient with THN and oncological diseases the profile of safety Zarsio® did not differ in
from those at adults. Safety and efficiency of use of medicine by the newborn are not established.
Recommendation about dosing for patients of children's age same, as for the adults receiving myelosuppressive cytotoxic chemotherapy.
DriversResearches were not conducted by
. Overdose to
Symptoms of overdose of Zarsio® are unknown to
. In 1-2 days after the treatment termination the quantity of the circulating neutrophils usually decreases by 50%, and in 1-7 days returns to norm. Side effects
Most frequent by-effects against the background of therapy using a filgrastim is bone and muscles pain from low to moderate severity (it is observed at 10% of patients). Bone and muscles pain, as a rule, is eliminated at intake of standard anesthetics.
At mobilization of PSKK at healthy donors frequent side reaction was skeletal and muscular pain.
Interaction
Safety and efficiency of administration of the medicine Zarsio® on the same day, as myelosuppressive cytotoxic khimiopreparat, are not established. Because of sensitivity of myeloid cells which quickly share, in myelosuppressive cytotoxic chemotherapy it is not recommended to appoint Zarsio® in the range of the 24th hour to or after administration of these medicines.
Storage conditionsto Store
at a temperature of 2 ° - 8 °C in original packing. Not to freeze. to Store
out of children's reach.
solution is stableAfter cultivation within 24 hours at a temperature of 2 ° - 8 °C. From the microbiological point of view solution should be used immediately.
Specifications
| Characteristics | |
| Active ingredients | Filgrastim |
| Applicant | Sandoz |
| Code of automatic telephone exchange | Filgrasty L03AA02 |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | The branded generic |
| Origin | Biological |
| Prescription status | According to the prescription |
| Primary packing | syringe |
| Producer | SANDOZ GMBH-TEHOPS |
| Quantity in packing | 5 syringes |
| Release form | solution for injections and infusions |
| Route of administration | Intravenous |
| Sign | Import |
| Storage temperature | from 2 °C to 8 °C |
| Trade name | Zarsio |















































