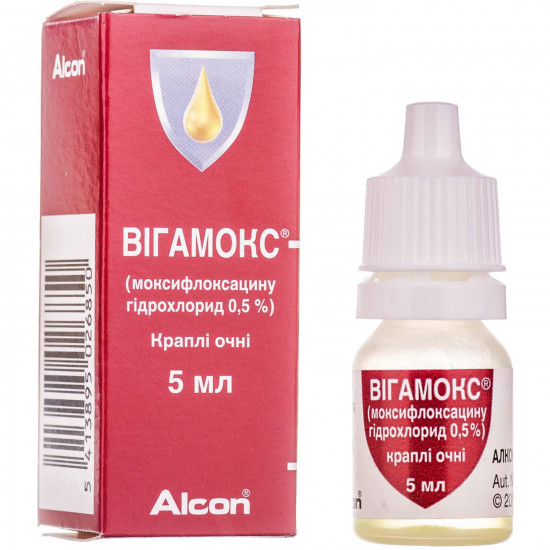








- Stock: In Stock
- Model: 179598
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Vigamoks cap. eye. 0.5% fl. 5 ml
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Drops eye Vigamoks of 0.5% are shown for topical treatment of the bacterial conjunctivitis caused by strains of bacteria, sensitive to a moksifloksatsin. For obtaining information of rather corresponding use of antibacterial agents you watch official installations.
Structure
Active ingredient: 1 ml of a moksifloksatsin of a hydrochloride of 5.45 mg that is equivalent to 5 mg of a moksifloksatsin;
Excipients: sodium chloride, boric acid, sodium hydroxide and/or the hydrochloric acid concentrated (for regulation rn), the water purified.
Contraindication
Hypersensitivity to active agent, other hinolon or to any of medicine components.
Route of administration
For ophthalmologic application.
Adult, including patients of advanced age
to Dig in on 1 drop in the affected eye (eyes) 3 times a day.
Usually the state improves within 5 days then it is necessary to continue treatment even the next 2-3 days. If within 5 days of the carried-out treatment of improvement it is not observed, it is necessary to consult with the doctor for specification of the diagnosis and/or treatment. Duration of treatment depends on severity of a disease and on a clinical and bacteriological picture of a course of the disease.
Feature of application
Pregnant
Vigamoks should not be applied during pregnancy unless the potential advantage of use of medicine exceeds potential risk for a fruit.
Children
during clinical trials of Vigamoks, it was to children, safe at application, including newborns.
Drivers
With care.
Overdose
Considering characteristics of medicine, the toxic effect in case of overdose at use of medicine in eyes is not expected or at accidental swallowing contents of one bottle.
Side effects
during clinical trials by the most widespread side reactions were eye pain and irritation of an eye which arose approximately in 1% - 2% of patients.
Interaction
byIf are at the same time appointed by several ophthalmologic medicines for topical administration, the interval between their applications has to make not less than 5 minutes. Oculentums should be applied the last.
Storage conditionsSpecial storage conditions of medicine are not provided by
. to Store
in the places inaccessible for children.
Expiration date - 3 years.
Specifications
| Characteristics | |
| Active ingredients | Moxifloxacin |
| Amount of active ingredient | 5 mg/ml |
| Applicant | Alcon |
| Code of automatic telephone exchange | S01AE07 Moxifloxacin |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | The branded generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | bottle |
| Producer | ALKON-KUVROR |
| Quantity in packing | 5 ml |
| Release form | eye drops |
| Route of administration | Eye |
| Sign | Import |
| Storage temperature | from 2 °C to 25 °C |
| Trade name | Vigamoks |
















































