


- Stock: In Stock
- Model: 176685
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Sterofundin ISO solution for inf. Comte. 500 ml No. 10
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
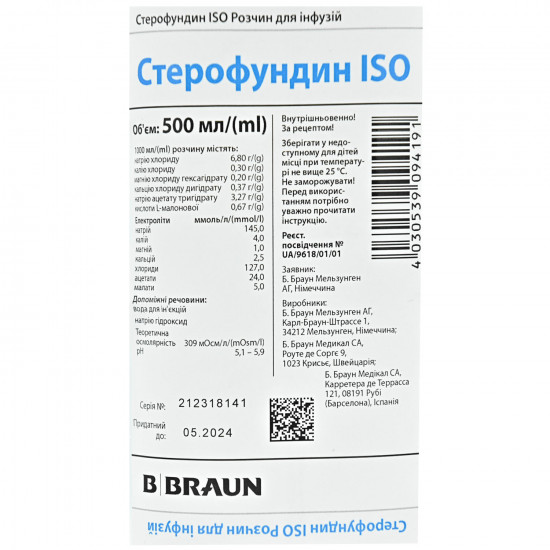
Solution for infusions "Sterofundin ISO" is applied to substitution of losses of intercellular liquid in case of isotonic dehydration at existence or threat of acidosis.
Structure
1000 ml of solution is contained (active ingredient):
- sodium of chloride of 6.80 g;
- potassium of chloride of 0.30 g;
- magnesium of chloride of hexahydrate of 0.20 g;
- Calcii chloridum dihydrate of 0.37 g;
- sodium of acetate of trihydrate of 3.27 g;
- acid of L-low-new 0.67 g
Concentration of electrolytes:
- sodium - 145 mmol/l;
- potassium - 4 mmol/l;
- magnesium - 1 mmol/l;
- calcium - 2.5 mmol/l;
- chlorides - 127 mmol/l;
- acetates - 24 mmol/l;
- malate - 5 mmol/l.
Excipients: water for injections, sodium hydroxide.
Contraindication
- hypersensitivity to any acting or to excipient which is a part of medicine;
- overhydratation;
- heavy stagnant heart failure;
- a renal failure with an oliguria or an anury;
- heavy general hypostasis;
- a hyperpotassemia in a severe form;
- hypercalcemia;
- metabolic Alkalosis;
- heavy metabolic acidosis.
Route of administration
Dose should be determined bydepending on the real need for replenishment of water level and electrolytes.
Maximum daily dose
Volume of the entered solution should not exceed 40 ml/kg of body weight a day (that corresponds to 5.8 mmol of sodium on 1 kg of body weight and 0.16 mmol of potassium on 1 kg of body weight).
Additional losses of liquid (for example in connection with fever, diarrhea, vomiting and so on) should be compensated todepending on the volume and composition of the lost liquid. In case of dehydration the dose of 40 ml/kg of body weight a day can be exceeded.
Dose should be calculated taking into account weight of dehydration and a clinical condition of the patient.
byAt treatment of the acute shortage of liquid namely expressed or life-threatening, hypovolemic shock, allows application of high doses, for example, by fast infusion (under pressure).
Maximum speed of infusion
Speed of infusion of medicine should not exceed 100 ml/hour
At treatment of dehydration the maximum speed of infusion is 5 ml/kg of body weight an hour that corresponds to 0.7 mmol of sodium on 1 kg of body weight an hour and 20 µmol of potassium on 1 kg of body weight in hour
At short-term replenishment of intravascular volume the maximum speed of infusion depends on a clinical situation of the patient.
In situations, life-threatening, it is possible to enter quickly 500 ml of medicine under manual pressure.
Solvent dosing and speed of infusion determineby
At use of the medicine "Sterofundin of ISO" as solvent mainly on the basis of characteristics and the mode of dosing of the dissolved means.
Pediatric population
appoints the Dose bydoctor. The dose depends on age, body weight, laboratory indicators, a clinical condition and the accompanying therapy of the patient.
Maximum daily dose
should not exceed the following daily doses (age):
- 28 days of life - 160 ml/kg of body weight a day;
- 2 months - 150 ml/kg of body weight a day;
- 1-2 years - 120 ml/kg of body weight a day;
- 3-5 years - 100 ml/kg of body weight a day;
- 6-12 years - 80 ml/kg of body weight a day;
- 13-18 years - 70 ml/kg of body weight a day.
depending on the volume and composition of the lost liquid.
in case of dehydration or at short-term replenishment of intravascular volume the above-stated doses can be raised.
Dose should be calculated taking into account weight of dehydration and a clinical condition of the patient.
infusion Speed
Maximum speed of infusion (body weight):
- 0-10 kg - 4 ml/kg/h;
- 10-20 kg - 40 ml/hour + 2 ml/kg/h are higher than 10 kg;
- > 20 kg - 60 ml/hour + 1 ml/kg/h is higher than 20 kg.
At treatment of dehydration the maximum speed of infusion is 5 ml/kg/h that corresponds to 0.7 mmol of sodium on 1 kg of body weight an hour and 20 µmol of potassium on 1 kg of body weight in hour
Method of administration
Only for introduction by infusion.
"Sterofundin of ISO" can be entered into peripheral veins (on pH and theoretical osmolarity).
for introduction needs to remove withAt introduction by fast infusion under pressure all air before infusion as otherwise there is a risk of developing of an air embolism during infusion from a plastic container and a system.
needs to carry out byAt introduction monitoring of liquid balance, plasma concentration of electrolytes and pH.
"Sterofundin of ISO" can be entered while there are indications for liquid substitution.
Feature of application
Pregnant
Data on use of the medicine "Sterofundin of ISO" pregnant and nursing, no. Within the recommended indications it is not necessary to expect any risk if the volume of the entered solution, the level of electrolytes and acid-base indicators are carefully controlled.
"Sterofundin of ISO" should be applied with care at toxicosis of pregnant women.
Drivers
"Sterofundin ISO" does not influence or has insignificant impact on ability to drive the car or to work with mechanisms.
Overdose
Excess or too fast introduction of solution can lead to a water or sodium overload with increase in turgor of skin, venous stagnation and with edematization, especially in case of violations sodium removal by kidneys. In this case the additional hemodialysis can be required.
Treatment
Immediately to stop infusion. Further treatment depends on character and weight of symptoms and can include introduction of diuretics with frequent control of electrolytic balance, correction of an electrolytic and acid-base imbalance.
Side effects
Can appear signs of overdose.
Reaction of hypersensitivity, including a small tortoiseshell.
overhydratation, a fluid lungs, electrolytic frustration Is possible.
Though oral administration of salts of magnesium stimulates witha vermicular movement, after administration of magnesium of sulfate was in rare instances reported about paralytic intestinal impassability.
Side reactions can be connected bywith technology of introduction, including the febrile answer, infections in the injection site, local pain or local reactions, irritation of veins, the vein thrombosis or phlebitis extending from the site of input and an ekstravazation. Side reactions can be also connected with the medicines attached to solution, the nature of the added substances will define type of any other undesirable effects.
Storage conditionsStores
at a temperature not above 25 °C, out of children's reach. Not to freeze.
Expiration date - 3 years.
Expiration date after opening of a container. From the microbiological point of view medicament should be used right after opening. If solution is entered not at once, the person who uses this medicament is responsible for its appropriate storage before the following application which usually should not exceed 24 hours at a temperature of 2-8 °C if in the controlled and confirmed aseptic conditions solution was not restored/is divorced.
Specifications
| Characteristics | |
| Active ingredients | Potassium chloride, Calcii chloridum, L-low-new Acid, Magnesium chloride, Sodium acetate trihydrate, Sodium chloride |
| Applicant | B. Braun |
| Code of automatic telephone exchange | B05BB01 Electrolytes |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | Traditional |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | container |
| Producer | B. BRAUN MELZUNGEN AG |
| Quantity in packing | 10 containers on 500 ml |
| Release form | solution for infusions |
| Route of administration | Infusional |
| Sign | Import |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Sterofundin |



















































