

- Stock: In Stock
- Model: 182885
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Niksar of the tab. of 20 mg No. 30
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Structure and form of release
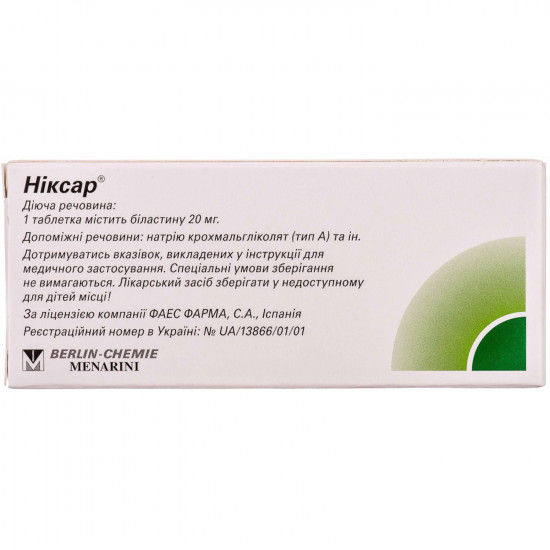
Active ingredient: bilastin.
Structure
- 1 tablet supports a belastin of 20 mg;
- additional substances: microcrystalline cellulose, sodium krakhmalglikolit (type A), silicon dioxide colloidal anhydrous, magnesium stearate.
release Form
Tablet.
Pharmacological properties
Pharmacodynamics. Bilastin is an antihistamine of long action, does not cause sedation, selectively contacts peripheral h1-receptors and does not contact m-holinoretseptorami.
After single use bilastin during 24 h is suppressed by development of the skin reactions caused by a histamine with blisters and an erythema.
during clinical trials in which patients with an allergic rinokonjyunktivit participated (seasonal and year-round) against the background of use of a bilastin in a dose of 20 mg of 1 times a day within 14–28 days discharges from a nose noted simplification of such symptoms of a disease as sneezing, an itching of a nose, congestion of a nose, an itching of eyes, dacryagogue and reddening of eyes. The medical effect of a bilastin remained during 24 h
during clinical trials in which patients with a chronic idiopathic small tortoiseshell took part, against the background of use of a bilastin in a dose of 20 mg of 1 times a day within 28 days noted reduction of severity of an itching and reduction of quantity and the size of bubbles; besides, patients felt the smaller discomfort caused by a small tortoiseshell. At patients the improvement of a dream and health was observed.
clinically significant lengthening of an interval of Q-T on the ECG, other disturbances from a cardiovascular system was not observed during the conducted clinical trials of a bilastin.
Pharmacokinetics. Absorption. After intake bilastin it is quickly soaked up, and its C max in blood plasma is reached in 1.3 h after reception. Accumulation of medicament in an organism is not revealed. The average rate of bioavailability at its oral administration is 61%.
Distribution. According to the researches in vitro and in Vivo, bilastin is substrate of a P-glycoprotein and protein carrier of OATP. Bilastin, obviously, is not substrate of the carrier of BCRP or the carrier of the industry standard, OAT1 and OAT3. Data of the researches in vitro do not give the grounds to consider what in a system blood-groove bilastin suppresses activity of such proteins carriers as P-gp, MRP2, BCRP, BSEP, OATP1B1, OATP1B3, OATP2B1, OAT1, OAT3, OCT1, OCT2 and NTCP as its ability to inhibit P-gp, OATP2B1 and OCT1 insignificant and is characterized by IC50 ≥300 value of micron that significantly exceeds a calculated value of the C max in blood plasma in case of its clinical use. Thus, similar interactions are clinically not significant. However results of similar researches indicate that inhibition bilastiny the proteins carriers which are in a mucous membrane of intestines (for example P-gp), it is impossible to exclude. After use in therapeutic doses bilastin contacts proteins of blood plasma for 84–90%.
Biotransformation. During the researches in vitro bilastin did not show ability to induce or suppress activity of isoenzymes of P450 cytochrome.
Removal. According to the research conducted with participation of volunteers after single use 14 C-bilastina in a dose of 20 mg of nearly 95% of the entered dose turned out in urine and Calais (28.3 and 66.5% respectively) in the form of not changed bilastin from what it is possible to draw a conclusion that in a human body bilastin it is metabolized slightly. On average T ½ bilastina at healthy volunteers makes 14.5 h
Linearity. In the range of doses from 5 to 220 mg the pharmacokinetic parameters of a bilastin change in direct ratio to a dose; at the same time at different healthy volunteers they differ slightly.
Indication
Symptomatic treatment at an allergic rinokonjyunktivit (seasonal and year-round) and a small tortoiseshell.
Use
Adults and children (aged from 12 years). The recommended dose makes 20 mg (1 tablet) of 1 times a day for relief of symptoms of the allergic rinokonjyunktivit (seasonal and year-round) and small tortoiseshells.
should take the Pill inside for 1 h to or in 2 h after meal or fruit juice.
Patients of advanced age. It is not required to patients of advanced age of dose adjustment. Experience of use of medicament for patients is aged more senior than 65 years is limited.
Renal failure. With renal failures of dose adjustment it is not required to patients.
Abnormal liver function. Experience of clinical use of medicament at patients with abnormal liver functions is absent. As bilastin is not exposed to metabolism and it is allocated mainly with kidneys, the abnormal liver function should not lead to increase in its systemic action up to dangerous level. Therefore with abnormal liver functions of dose adjustment it is not required to patients.
treatment Duration. Patients with allergic rhinitis should use medicament only during contact with allergens. Persons with seasonal allergic rhinitis can stop treatment after reduction of expressiveness of symptoms and to renew after their return. Patients with year-round allergic rhinitis can use medicament continuously during the period of contact with allergens. At patients with a small tortoiseshell duration of therapy depends on character and duration of symptoms and also on their dynamics.
Contraindication
Hypersensitivity to active ingredient (bilastin) or to any of excipients.
Side effects
during clinical trials at patients with an allergic rinokonjyunktivit or a chronic idiopathic small tortoiseshell the side effects against the background of use of a bilastin in a dose of 20 mg arose approximately with the same frequency, as against the background of placebo use (12.7 and 12.8%). results of the conducted researches demonstrate that at use of a bilastin in a dose of 20 mg most often noted such side reactions as a headache, drowsiness, dizziness and fatigue. approximately with a similar frequency these undesirable phenomena revealed at the patients accepting placebo.
bygave Below side reactions which communication with bilastiny is recognized or, at least, is possible and which in the program of clinical development were noted at more than 0.1% of the patients receiving bilastin in a dose of 20 mg.
side effects are distributed byOn frequency on the following categories: very often (≥1/10); often (≥1/100 and 1/10); sometimes (≥1/1000 and 1/100); seldom (≥1/10,000 and 1/1000); very seldom (1/10,000), it is unknown (the available data do not allow to give an assessment).
Infection and parasitic diseases: sometimes — oral cavity herpes.
Disturbance of food and metabolism: sometimes — the increased appetite.
from mentality: sometimes — uneasiness, insomnia.
from an organ of hearing and balance: sometimes — sonitus, dizziness.
from a cardiovascular system: sometimes — blockade of the right leg of a ventriculonector, a sinus arrhythmia, lengthening of an interval of Q-T on the ECG, other disturbances on the ECG.
from nervous system: often — drowsiness, a headache; sometimes — dizziness.
from a respiratory system: sometimes — an asthma, unpleasant feelings in a nose, dryness in a nose.
from digestive system: sometimes — pain at the top of a stomach, an abdominal pain, nausea, unpleasant feelings in a stomach, diarrhea, dryness in a mouth, dyspepsia, gastritis.
from skin and hypodermic fatty tissue: sometimes — an itching.
General and local disturbances: sometimes — fatigue, thirst, strengthening of already available diseases, fever, an asthenia.
Additional methods of a research: increase in activity gamma glutamiltranspeptidazy, AlAT, AsAT, increase in level of creatinine in blood, increase in the TG level in blood, increase in body weight.
Special instructionsAt patients with moderate or heavy renal failures the use of a bilastin along with p-glycoprotein inhibitors (ketokonazol, erythromycin, cyclosporine, ritonavir, diltiazem, etc.) can lead
to increase in concentration of a bilastin in blood plasma and, therefore, to increase in risk of its side effects. therefore patients with moderate or heavy renal failures bilastin along with inhibitors of a p-glycoprotein should not apply.
Use during pregnancy and feeding by a breast. Fertility. Clinical data are limited. Researches on animals of negative impact of medicament on fertility did not reveal.
Pregnancy. Data on use of a bilastin for pregnant women are absent.
byduring the researches on animals also noted harmful effects of medicament (direct or indirect) on reproductive function, childbirth or post-natal development. Taking into account safety the medicament Niksar during pregnancy should not be used.
Feeding by a breast. Data on whether gets bilastin into breast milk of women, no. Discharges of a bilastin with milk at animals did not study. The decision on extension or the termination of breastfeeding and also on continuation or the termination of therapy by the medicament Niksar should be accepted taking into account advantage of breastfeeding for the child, on the one hand, and need of therapy bilastiny for mother — with another.
Children. Safety and efficiency of a bilastin for children aged up to 12 years are not confirmed.
Ability to influence speed of response at control of vehicles or work with other mechanisms. According to a research of influence of a bilastin on ability to run vehicles, the use of a bilastin in a dose of 20 mg does not affect ability to run vehicles. However patients should be informed that in some cases medicament can cause drowsiness and, thus, affect ability to run vehicles and to work with mechanisms.
Interaction
Interaction with foodstuff. food reduces bioavailability of the bilastin accepted inside by 30%.
Interaction with grapefruit juice. In case of use of a bilastin in a dose of 20 mg along with grapefruit juice the bioavailability of a bilastin decreased by 30%. The similar effect can be observed also in case of use of other fruit juice. Extent of decrease in bioavailability can differ depending on producer of juice and fruit from which it is received. This interaction is caused by ability of components of fruit to suppress activity of protein — the carrier of organic OATP1A2 anions for whom bilastin is substrate. Also medicines which are substrates or OATP1A2 inhibitors can reduce concentration of a bilastin in blood plasma (for example ritonavir or rifampicin).
Interaction with ketokonazoly or erythromycin. In case of reception of a bilastin along with ketokonazoly or AUC erythromycin of a bilastin the C max — by 2–3 times increased twice, and. Similar effects can be explained with interaction at the level of the proteins carriers which are responsible for removal of medicines from intestines cells as bilastin is substrate of a P-glycoprotein and it is not metabolized. On the one hand, and a ketokonazol or erythromycin, with another, these effects probably do not influence a profile of safety of a bilastin. Also other medicines which are substrates or inhibitors of a P-glycoprotein can increase concentration of a bilastin in blood plasma (for example cyclosporine).
Interaction with diltiazem. In case of reception of a bilastin in a dose of 20 mg along with diltiazem in a dose of 60 mg of the C max bilastina increased by 50%. The similar effect can be explained with interaction at the level of the proteins carriers which are responsible for removal of medicines from intestines cells; this effect probably does not influence a profile of safety of a bilastin.
Interaction with alcohol. After a concomitant use of alcohol and a bilastin the psychomotor functions were in a dose of 20 mg at the same level, as after a concomitant use of alcohol and placebo. Bilastin does not influence psychomotor functions at reception with alcohol.
Interaction with lorazepam. In case of use of a bilastin in a dose of 20 mg along with lorazepam in a dose of 3 mg for 8 days of strengthening of the oppressing effect of lorazepam on central nervous system it is not revealed.
OverdoseData concerning acute overdose are received by
only during clinical trials. against the background of use of a bilastin in the doses exceeding therapeutic at 10-11 times, side effects at healthy volunteers arose twice more often than against the background of placebo use. side effects which were noted more often included dizziness, a headache and nausea. serious side reactions and significant increase in duration of an interval of q-tc are noted. during the cross research with measurement of intervals of q-t/q-tc in which studied influence of repeated use of a bilastin on repolarization of ventricles the significant lengthening of an interval of q-tc is not revealed.
Treatment. In case of overdose the symptomatic and maintenance therapy is recommended. Specific antidote for a bilastin is unknown.
Storage conditions
does not demand special storage conditions.
Specifications
| Characteristics | |
| Active ingredients | Bilastin |
| Amount of active ingredient | 20 mg |
| Applicant | Berlin-Chemie Menarini |
| Code of automatic telephone exchange | R06AX29 Hifenadin |
| Interaction with food | To |
| Light sensitivity | Not sensitive |
| Market status | The branded generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | blister |
| Producer | FAYES PHARM S.A |
| Quantity in packing | 30 tablets (3 blisters on 10 pieces) |
| Release form | tablets for internal use |
| Route of administration | Oral |
| Sign | Import |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Niksar |

















































