






- Stock: In Stock
- Model: 187355
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Milistan Kombi a nasal Rapid test for definition of antigen of COVID-19 virus and flu of A/V of 1 piece
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Milistan Kombi the nasal Rapid test test for detection of antigen of COVID-19 virus and flu of A/V is an immunoassay of in vitro. The analysis is intended for direct and high-quality detection of antigens of virus SARS-CoV-2 nucleoprotein and flu of A/V samples of contents of a nasal cavity.
Principle of carrying out analysis
Test for COVID-19:
antiSARS-CoV-2 are immobilized byAntibody in test area of a nitrocellulose membrane. The antibodies of anti-SARS-CoV-2 conjugated with the painted particles are immobilized on a small pillow with a conjugate. The nasal discharges collected by the user have to be mixed with the buffer for extractions which is individually packed into set.
byduring testing target antigens if they are in nasal discharges, will be released in the buffer for extraction. Movement of a sample happens under the influence of capillaries, it interacts with reagents on a small pillow for a sample, then target antigens will contact antibodies of anti-SARS-CoV-2 on a small pillow for a conjugate. Thus, the antigen-antibody complex will take the antibodies of anti-SARS-CoV-2 immobilized in test area. The excess painted particles will be taken in control area of a membrane.
Existence of a color strip in a test zone indicatespositive take for antigens of virus SARS-CoV-2, and its absence means negative take. The color strip in an inspection zone is intended for the procedure of control, specifying that enough a sample was added and migration of liquid along a membrane took place successfully.
Test for A/V flu:
Antibody of an antivirus of flu A and an antibody of an antivirus of flu B are immobilized in two certain tested areas of a nitrocellulose membrane. The antibodies of an antivirus of flu A and an antibody of an antivirus of flu B conjugated with the painted particles, immobilized on the conjugated small pillow.
Nasal discharges collected by the user have to mix up with the buffer for extraction which is individually packed in set.
byduring testing target antigens if they are in nasal discharges, will be released in the buffer for extraction. The sample moves along a strip under the influence of capillaries, it interacts with reagents on a small pillow for a sample and then target antigens contact antibodies on a small pillow for a conjugate. Therefore, the antigen-antibody complex will be taken by the antibodies immobilized in two test areas. The excess painted particles will be taken in the control site of a membrane.
Existence of a color strip in test zones indicatespositive take for viral antigens of flu A and B, and its absence means negative take. The color strip in an inspection zone is intended for the procedure of control, specifying that enough a sample was added and migration of liquid along a membrane took place successfully.
Complete set
Materials provided
- handle for a fence of contents of a nasal cavity with test strips of COV and FLU;
- buffer for extraction;
- operation manual.
Necessary materials which are not provided
- hours, the timer or a stop watch.
Feature of use
- only for in vitro diagnostics;
- carefully insert the device into nostrils; not to swallow of
- ;
- before test attentively read the operation manual for obtaining the correct result. It is necessary to adhere to its instructions;
- not to use set or its components after an expiration date;
- device contains material of animal origin, with it it is necessary to handle as constituting potential biological danger. Not to use if packing is damaged or open; test devices are packed by
- into the foiled packages preventing penetration of moisture at storage. Before opening examine a package from a foil. Not to use the device if in a foil there are openings or if the package is not completely soldered. It is possible to receive false results if to use reagents or components which were stored in an inadequate way;
- not bringing samples and reagents to room temperature before testing can reduce sensitivity of the analysis. The inexact or wrong fence, storage and transportation of samples can result in false-negative results of the analysis;
- avoid contact of skin with the buffer;
- do not punch a foil in capacity with the buffer for extraction prior to testing.
Route of administration
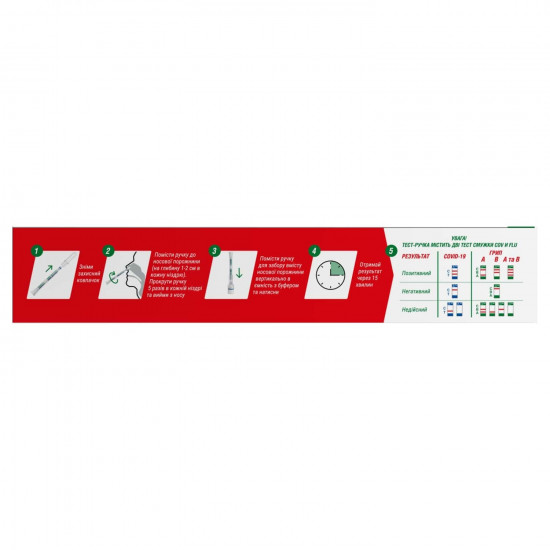
Procedure of test
Before use needs to bring test set to room temperature (15 ~ 30 °C).
Remove the handle for a fence of contents of a nasal cavity from packing, remove a protective cap from a small pillow for a sample fence.
Accurately insert a small pillow for a sample fence into the nasal course on depth about 1 - 2 cm, twist 5 (five) times and take.
Make similar manipulation with the second nasal course for a fence of enough a sample.
Place the test handle in capacity with the buffer for extraction, carefully press to break off a foil. The edge of capacity with the buffer for extraction has to match the upper edge of a marking ring on the test device.
Determine byresult in 15 minutes.
Important
needs to be received as the bigger quantity of a sample
is possible forThis procedure can lead to discomfort. Do not advance a small pillow further if strong resistance is felt. Testing of children from 2 to 15 years has to be held by adults.
Should watch that the marking ring on the test device matched edge of capacity with the buffer for extraction. Otherwise it will lead to disturbance of a lateral stream and invalid result of testing.
Result
POSITIVE
For tests for COVID-19. The test COV strip
- On a membrane two color strips appear. One strip appears in a zone of control (C), and other strip appears in a test zone (T).
For the test flu A. The test FLU strip
- One color strip appears in a zone of control (C). The second strip appears in test zone A (on bottom edge).
For the test flu B. The test FLU strip
- One color strip appears in a zone of control (C). The second strip appears in test zone B (in a membrane).
For the test for flu A and B. The test FLU strip
- One color strip appears in a zone of control (C). Two strips appear in a test zone.
NEGATIVE
For tests for COVID-19. The test COV strip
is displayed byIn a zone of control (C) only one color strip.
In a test zone (T) visible color strips do not appear.
For the test flu A and B. The test FLU strip
is displayed byIn a zone of control (C) only one color strip.
In a test zone (B) and (A) there are no visible color strips.
INVALIDResults of any test which did not give a strip in a control zone after the specified time for reading of results are invalid
. Check the procedure of the test and repeat it, using the new test. If the problem is not fixed, immediately stop use of set and address the local distributor.
efficiency Indicators
Analytical sensitivity:
Limit of detection (LOD) Milistan of Kombi of the nasal Rapid test test for detection of antigen of COVID-19 virus and flu of A/V defined as concentration of an influenza virus and the SARS-CoV-2 virus, the providing positive take of testing makes about 95% as it was defined by assessment of various concentration of the inactivated influenza virus And (H3N2, H1N1), the inactivated influenza virus In (Victoria, Yamagata) and the inactivated COMFORT virus SARS-CoV-2 MILISTAN the nasal Rapid test test for detection of antigen of COVID-19 virus and flu A / V. Bylo carried out 20 tests for each concentration. Results of testings defined concentration of 1.0×104 TCID50/mL as LOD for flu of A (H3N2), 4,3×104 TCID50 as LOD for influenza A (H1N1), 2,2×105 TCID50 for flu B (Victoria), 2, 5×105 TCID50 for flu B (Yamagata) and 1×102.4 TCID50/mL as LOD for SARS-CoV-2.
Clinical assessment:
For detection of antigen of COVID-19 virus:
All 197 clinical samples for check of efficiency Milistan as Kombi of the nasal Rapid test test for identification of antigen of COVID-19 virus and flu of A/V in comparison with ZT-PTsR testing were chosen p. No differences in results of testing depending on age and sex of the patient were revealed. 41 samples were found by the RT-PCR positive method, and 156 samples negative when testing by ZT-PTsR method. These samples were tested by means of MILISTANA KOMBI of the nasal Rapid test test for detection of antigen of COVID-19 virus and flu of A/V.
Storage conditions
to STORE MILISTAN the COMFORT the nasal Rapid test test for definition of antigen of COVID-19 virus when it is not used, it is necessary at a temperature of 2 ~ 30 °C.
be not frozen by.
Components of set are stabletill the expiry date specified on packing.
Specifications
| Characteristics | |
| Country | China |
| Producer | ASSURE TECH CO LTD |
| Quantity in packing | 1 pieces |
| Release form | test systems |

















































