






- Stock: In Stock
- Model: 184803
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Gabesat time. liof. for solution for inf. 100 mg fl. + r-nl of amp. 5 ml No. 1
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Gabesat – inhibitor of proteases for treatment of acute pancreatitis.
Structure
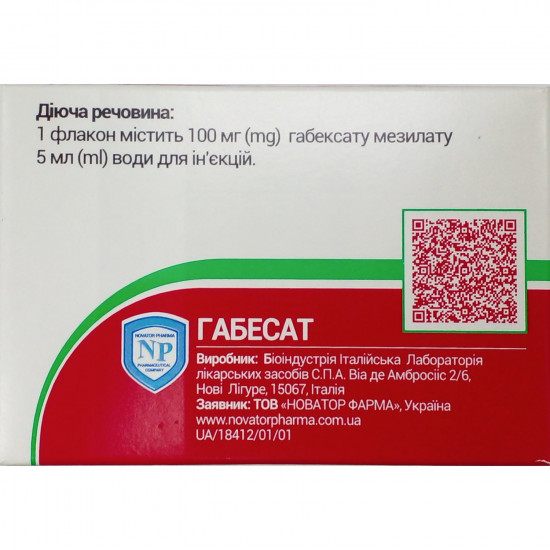
- active ingredient: 1 bottle contains 100 mg of the gabeksat of the mezilat;
- excipients: water for injections.
Contraindication
Hypersensitivity to active ingredient.
Side reactions
General disturbances and states concerning the place of an injection - heaktsii on the place of an injection: necrotic ulcers, phlebitis and fragility of a vein. Pain, redness. Hypersensitivity (skin rash, an itching, inflammation in the injection site). Fever, face reddening, shock (acute anaphylaxis, anaphylactic reactions, pressure decrease, precardiac oppression, short wind, a loss of consciousness, hypostasis of drinks / throat or change of health)
from a cardiovascular system – a lowering of arterial pressure.
from nervous system – a headache.
from digestive tract – nausea, vomiting.
from a haemo lymphopoietic system and fibrillation - a leukopenia, a granulocytopenia, the speeded-up bleeding.
Route of administration
to Begin treatment with 1-3 bottles a day (100-300 mg of Gabeksat of the mezilat) with intravenous infusion on one drop with a speed no more than 8 ml/minute, and then to reduce a dosage as the clinical picture has to improve. If necessary the above-stated dose can be increased by 1-3 bottles during the same day.
sterile powder which is Contained in a bottle needs to be dissolved solvent from a bottle (water for injections).
Received solution needs to be parted within addition in 500 ml of lactat Ringera solution or 5% of glucose. After preparation, solution needs to be used immediately, and to destroy the remains.
Solution for intravenous infusion should be entered slowly, regulating speed so that it did not exceed 8 ml a minute and 2.5 mg of the gabeksat of the mezilat for body kg in hour
Dosage has to be adjusted according to the patient's symptoms.
Feature of use
Use during pregnancy or feeding by a breast
for the confirmed or expected pregnancy a dosage of the gabeksat of the mezilat should be carried out in minimal effective doses and only according to indications, life-threatening the patient.
Data on any excretion in breast milk are absent.
Childrennot to use
to children. Ability to influence speed of response at control of motor transport or other mechanisms
during medicine use to patients should not run transport or to work with other mechanisms.
Overdose
in case of developing of headaches, reduction of partial tromboplastinovy time, appearance of bleeding, hypotension, nausea, vomiting, diarrhea, skin rashes, an itching or face reddening a dosage should be reduced.
If symptoms remain, it is necessary to stop use of medicine and to see a doctor.
toand other types of interactions
toDoes not know Interaction with other medicines.
At the accompanying therapy by other parenteral medicaments Gabesat should be entered separately.
Storage conditionsto Store
in the dry, protected from light place at a temperature not above 25 °C. To store out of children's reach.
Specifications
| Characteristics | |
| Active ingredients | Gabeksat mesylate |
| Amount of active ingredient | 200 mg |
| Code of automatic telephone exchange | B02AB49 Gabeksat |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | Generic-generic |
| Origin | Chemical |
| Prescription status | Without prescription |
| Primary packing | bottle |
| Producer | S.P.A. LABORATORIO ITALIANO MEDITSINALI'S BIOINDASTRIA. |
| Quantity in packing | 1 bottle |
| Release form | powder for infusion solution |
| Route of administration | Infusional |
| Sign | Import |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Gabesat |














































