

- Stock: In Stock
- Model: 182270
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Azomex tab. of 5 mg No. 30
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Pharmacological properties
Pharmacodynamics. amlodipin — racemic mix s (–) - and r (+) - isomers. s-amlodipine — an active chiral form of an amlodipin — a blocker of slow calcium channels; blocks receipt of calcium ions through membranes in cells of unstriated muscles of a myocardium and vessels. the mechanism of antihypertensive effect of medicament azomex is caused by direct influence on unstriated muscles of vessels.
Anti-anginal effect of an amlodipin is implemented by two ways.
1. Amlodipin expands peripheral arterioles and thus reduces OPSS and an afterload. As ChSS at the same time practically does not change, load of heart, consumption of energy and the need of a myocardium for oxygen decreases.
2. Expansion of the main coronary arteries and coronary arterioles (normal and ischemic), perhaps, also plays a role in the mechanism of action of an amlodipin. Such expansion increases saturability of a myocardium oxygen at patients with a spasm of a coronary artery (Printsmetal's stenocardia or alternative stenocardia).
single dose of an amlodipin provides toAt patients with AG clinically significant decrease in the ABP for 24 h that allows to apply it 1 time a day. Thanks to the slow beginning of action amlodipin does not cause acute arterial hypotension.
At patients with stenocardia at use of 1 daily dose the general time of physical activity, time prior to the beginning of stenocardia and time increases to development of 1 mm of a depression of a segment of ST. Drug reduces the frequency of attacks of stenocardia and the need for nitroglycerine use. Amlodipin has no adverse impact on a metabolism and level of lipids in blood plasma therefore he is suitable for treatment of patients OH, diabetes, gout.
Pharmacokinetics
Absorption/distribution. After intake in therapeutic doses amlodipin it is well soaked up, reaching the C max in blood through 6–12 h. The absolute bioavailability by calculations is 64–80%. The volume of distribution is about 21 l/kg. Meal does not influence absorption of an amlodipin.
Metabolism/removal. T ½ makes about 35-50 h of blood plasma that provides a possibility of prescribing of medicament of 1 times a day. Resistant equilibrium concentration in plasma is reached in 7–8 days of regular use of drug. Amlodipin is transformed in a liver with formation of inactive metabolites; 10% of not changed medicament and 60% of metabolites are removed with urine.
Patients of advanced age. Time of achievement of equilibrium concentration of an amlodipin in blood plasma like what is observed at patients of advanced age and at adult patients. The clearance of an amlodipin is usually a little reduced that at patients of advanced age leads to increase in AUC and T ½ drug.
Patients with a renal failure. Amlodipin is extensively metabolized to inactive metabolites. 10% of an amlodipin are removed in not changed view with urine. Changes of concentration of an amlodipin in blood plasma do not correlate with degree of a renal failure. Patients with a renal failure can apply amlodipin in usual doses. Amlodipin does not leave by dialysis.
Patients with an abnormal liver function. Information on use of an amlodipin for patients with an abnormal liver function is very limited. At patients with a liver failure the clearance of an amlodipin is reduced that leads to increase in duration of T ½ and to increase in AUC approximately for 40–60%.
Indication
Ag. ischemic heart disease: stable stenocardia of loading, vasospastic stenocardia (including stenocardia printsmetat).
Use
Pri ag and stenocardia the initial dose of an azomex makes 2.5 mg of 1 times a day; if necessary this dose can be raised to 5 mg of 1 times a day depending on individual reaction of the patient.
Children. Safety and efficiency of use of medicament for children are not established therefore it is not applied in pediatric practice.
Contraindication
- Known hypersensitivity to dihydropyridines, an amlodipin or any other component of drug; arterial hypotension of heavy degree; shock (including cardiogenic); obstruction of the taking-out path of a left ventricle (for example a stenosis of an aorta of heavy degree); hemodynamically unstable heart failure after an acute myocardial infarction.
Side effects
by
At use of an amlodipin a thicket it was reported about such side reactions as drowsiness, dizziness, a headache, palpitation, inflows, an abdominal pain, nausea, hypostases of shins, hypostases and fatigue.
Side reactions about which it was reported during use of an amlodipin are given bybelow on systems and classes of bodies and for emergence frequency: very often (≥1/10), it is frequent (≥1/100–1/10), infrequently (≥1/1000– ≤ 1/100), is rare (≥1/10 000– ≤ 1/1000), is very rare (≤1/10,000).
from blood and lymphatic system: very seldom — a leykotsitopeniya, thrombocytopenia.
from the immune system: very seldom — allergic reactions.
from metabolism and alimentary disorders: very seldom — a hyperglycemia.
Mental disturbances: infrequently — insomnia, changes of mood (including uneasiness), a depression; seldom — confusion of consciousness.
from nervous system: often — drowsiness, dizziness, a headache (mainly in an initiation of treatment); infrequently — a tremor, a dysgeusia, a syncope, a hypesthesia, paresthesia; very seldom — a hyper tone, peripheral neuropathy.
from an organ of sight: infrequently — a disorder of vision (including a diplopia).
from an organ of hearing and a labyrinth: infrequently — a ring in ears.
from heart: infrequently — the strengthened heartbeat; very seldom — a myocardial infarction, arrhythmia (including bradycardia, ventricular tachycardia and fibrillation of auricles).
from vessels: often — inflows; infrequently — arterial hypotension; very seldom — a vasculitis.
Respiratory, thoracic and mediastinal disturbances: infrequently — dispnoe, rhinitis; very seldom — cough.
from digestive system: often — an abdominal pain, nausea; infrequently — vomiting, dyspepsia, disturbance of a vermicular movement of intestines (including a constipation and diarrhea), dryness in a mouth; very seldom — pancreatitis, gastritis, a hyperplasia of gums.
from a gepatobiliarny system: very seldom — hepatitis, jaundice, increase in level of liver enzymes (that most often was associated with a cholestasia).
from skin and hypodermic fabric: infrequently — an alopecia, a purpura, change of coloring of skin, the increased sweating, an itching, rash, a dieback; very seldom — a Quincke's disease, a multiformny erythema, a small tortoiseshell, exfoliative dermatitis, Stephens's syndrome — Johnson, a Quincke's edema, photosensitivity.
from skeletal and muscular and connecting fabrics: often — swelling of shins; infrequently — an arthralgia, myalgia, myotonia, a dorsodynia.
from kidneys and an urinary path: infrequently — urination disturbance, a nocturia, the increased urination frequency.
from a reproductive system and mammary glands: infrequently — impotence, a gynecomastia.
General disturbances and a state in the injection site: often — hypostasis, fatigue; infrequently — pain behind a breast, an asthenia, pain, an indisposition.
Research: infrequently — increase or degrowth of a body.
toIt was reported about exceptional cases of development of an extrapyramidal syndrome.
Message about the suspected side reactions. Messages about the suspected side reactions after registration of medicine are important. It allows to carry out continuous monitoring of a ratio between the advantage and risks connected with use of this drug. Doctors should report on any suspected side reactions according to requirements of the legislation.
Special instructionsSafety and efficiency of use of an amlodipin in hypertensive crisis were not estimated by
.
Patients with heart failure. At this category of patients amlodipin it is necessary to apply with care. At patients with heart failure of heavy degree (class III and IV on classification of NYHA) at use of an amlodipin the frequency of cases of edematization of lungs was higher in comparison with placebo use. Patients with stagnant heart failure have blockers of calcium channels, including amlodipin, it is necessary to apply with care as they can increase risk of cardiovascular events and lethal cases in the future.
Patients with an abnormal liver function. T ½ an amlodipina and the AUC parameters higher at patients with an abnormal liver function; there are no recommendations concerning a dose of drug. Therefore at this category of patients the use of medicament should be begun with the lowest dose. It is necessary to be careful both at the beginning of medicament use, and at increase in a dose. Slow selection of a dose and careful observation of their state can be required by patients with a heavy liver failure.
Patients of advanced age. It is necessary to raise a medicament dose at this category of patients with care.
Patients with a renal failure. At this category of patients it is necessary to apply usual doses of drug. Changes of concentration of an amlodipin in blood plasma do not correlate with extent of disturbances of functions of kidneys. Amlodipin is not brought by dialysis.
Amlodipin does not affect results of laboratory researches.
is not recommended to be applied amlodipin together with grapefruit or with grapefruit juice as at some patients the bioavailability can be increased that will lead to strengthening of hypotensive effect of drug.
Use during pregnancy and feeding by a breast. Safety of use of an amlodipin during pregnancy is not established. It is recommended to apply amlodipin during pregnancy only when there is no safer alternative, and the risk connected with the disease exceeds possible harm from treatment for mother and a fruit.
byduring the researches on animals at use of high doses observed reproductive toxicity.
feeding Period breast. It is unknown whether gets amlodipin into breast milk. At making decision on feeding continuation by a breast or use of an amlodipin it is necessary to estimate advantage of breastfeeding for the child and advantage of use of medicament for mother.
Fertility. It was reported about reversible biochemical changes of a head of a spermatozoon at some patients when using blockers of calcium channels. Clinical information on potential influence of an amlodipin on fertility is not enough.
Ability to influence speed of response at control of vehicles or work with other mechanisms. Amlodipin can have insignificant or moderate impact on ability to run vehicles or to work with other mechanisms. Speed of response can be reduced in the presence of such symptoms as dizziness, a headache, increased fatigue or nausea. It is necessary to be careful, especially at the beginning of therapy.
Interaction
Influence of other medicines on amlodipin
CYP Inhibitors 3A4. Simultaneous use of an amlodipin and CYP inhibitors 3A4 of powerful or moderate action (inhibitors of proteases, azolny antifungal means, macroleads, such as erythromycin or klaritromitsin, verapamil or diltiazem) can lead to significant increase in exposure of an amlodipin that can also result in the increased risk of developing of hypotension. Patients of advanced age can have more expressed clinical value of such changes. Clinical observation of a condition of the patient and selection of a dose can be required.
todoes not recommend simultaneous use of an amlodipin and consumption of grapefruit or grapefruit juice as at some patients the bioavailability of an amlodipin can increase that, in turn, will lead to strengthening of hypotensive effect.
Klaritromitsin. Klaritromitsin is CYP inhibitor 3A4. There is an increased risk of developing of hypotension at patients who accept klaritromitsin with amlodipiny. At simultaneous use of an amlodipin with klaritromitsiny it is recommended to carry out careful control of a condition of patients.
Inductors CYP 3A4. There is no information concerning influence of inductors CYP 3A4 on amlodipin. Simultaneous use of an amlodipin and the substances which are inductors CYP 3A4 (for example rifampicin, St. John's wort extract), can lead to decrease in concentration of an amlodipin in blood plasma therefore it is necessary to apply such combinations with care.
Dantrolen (infusions). At animals developed ventricular fibrillation with a lethal outcome and the cardiovascular collapse associated with a hyperpotassemia after use of verapamil and a dantrolen in / century. Because of risk of development of a hyperpotassemia it is recommended to avoid use of blockers of calcium channels, such as amlodipin, at patients, inclined to a malignant hyperthermia, and at treatment of a malignant hyperthermia.
Influence of an amlodipin on other medicines. The hypotensive effect of an amlodipin exponentiates hypotensive effect of other antihypertensive medicines.
Takrolimus. There is a risk of increase in levels of a takrolimus in blood at simultaneous use with amlodipiny, however the pharmacokinetic mechanism of such interaction is completely not installed. To avoid toxicity of a takrolimus at simultaneous use of an amlodipin, regular monitoring of level of a takrolimus in blood and, if necessary, dose adjustment is necessary.
Cyclosporine. Researches of interaction of cyclosporine and an amlodipin were not conducted at healthy volunteers or other groups of the population, except for patients with a transplantirovanny kidney at whom noted increase in residual concentration of cyclosporine (on average 0–40%). Therefore at patients is after transplantation of the kidneys applying Azomex it is necessary to carry out monitoring of level of cyclosporine and in case of need the dose of the last has to be reduced.
Simvastatin. Simultaneous repeated use of 10 mg of an amlodipin from 80 mg of a simvastatin led to 77% to increase in exposure of a simvastatin in comparison with monotherapy simvastatiny. Therefore the dose of a simvastatin should not exceed 20 mg/days for the patients taking this medicament combined with amlodipiny.
Overdose
Experience of intentional overdose of medicament is limited.
overdose Symptoms: the available information gives the grounds to consider that the considerable overdose of medicament will lead to an excessive peripheral vazodilatation and, perhaps, to reflex tachycardia. It was reported about development considerable and, perhaps, long system hypotension, including shock with a lethal outcome.
Treatment: clinically significant arterial hypotension caused by overdose of an amlodipin demands active support of activity of a cardiovascular system, including frequent monitoring of functions of heart and breath, a raising of extremities, monitoring of volume of the circulating liquid and urination.
For restoration of a tone of vessels and the ABP can use vasoconstrictive drugs, having made sure of lack of contraindications to their use. Gluconate calcium use in/in can be useful for leveling of effects of blockade of calcium channels.
In certain cases useful. Use of activated carbon for healthy volunteers for 2 h after introduction of 10 mg of an amlodipin considerably reduced the level of its absorption.
As amlodipin highly contacts proteins, the effect of dialysis is insignificant.
Storage conditions
In original packing at a temperature not above 25 °C.
Specifications
| Characteristics | |
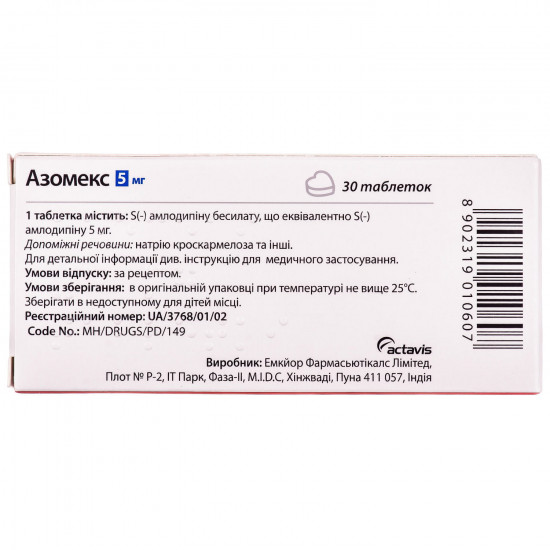
| Active ingredients | Amlodipin |
| Amount of active ingredient | 5 mg |
| Applicant | Teva |
| Code of automatic telephone exchange | C08CA01 Amlodipin |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | The branded generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | blister |
| Producer | EMKYOR PHARMASYYUTIKALS LIMITED |
| Quantity in packing | 30 tablets (3 blisters on 10 pieces) |
| Release form | tablets for internal use |
| Route of administration | Oral |
| Sign | Import |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Azomex |



















































