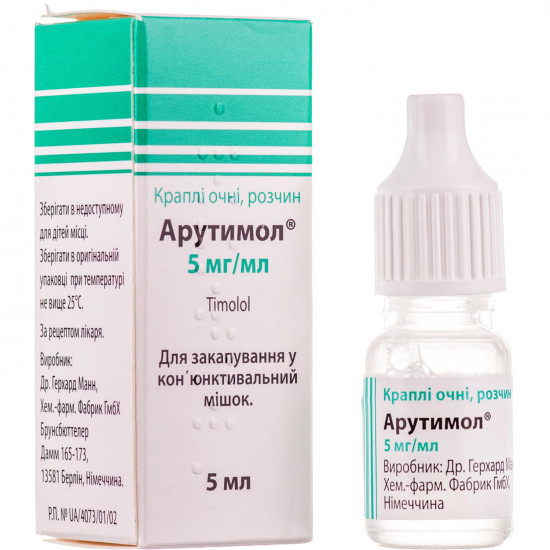






- Stock: In Stock
- Model: 179704
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Arutimol cap. eye. 5mg/ml fl. 5 ml No. 1
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Pharmacological properties
Pharmacodynamics. Timololum is a non-selective blocker of β-adrenoceptors which does not render sympathomimetic or mestnoanesteziruyushchy (membrane stabilizing) action. Timololum inhibits β1-рецепторы which are localized mainly in a cardiac muscle and also β2-рецепторы.
Timololum reduces the stimulating effect of catecholamines on heart. As a result the conductivity of an AV node slows down, ChSS and minute volume of heart decreases. Blockade of β-adrenoceptors in bronchial tubes and bronchioles leads to increase in resistance of airways due to the lack of counteraction for parasympathetic activity.
Local action on eyes. Eye drops Arutimol reduce as raised, and normal intraocular pressure.
Exact mechanism of effect of Timololum which is shown in decrease in intraocular pressure is unknown to. However fluorofotometrichesky and tonografichesky researches demonstrate that the effect of Timololum can be caused by reduction of secretion of watery moisture. Some researches also showed improvement of outflow of watery moisture. At some patients after long-term treatment the decrease in sensitivity to Timololum, as well as to other medicaments reducing intraocular pressure was noted. Long-term researches with participation of 164 patients investigated at least 3 years showed that after achievement of stabilization of intraocular pressure there were no its any serious changes. Unlike miotocs, Timololum reduces intraocular pressure, without influencing accommodation and the size of a pupil. The absence of a miosis is especially important for patients with a cataract. In case of transition of the patient from use of miotocs to Timololum it is necessary to carry out correction of a refraction after reduction of miotic action.
Pharmacokinetics. Effect of medicine comes, as a rule, in 20 min. after topical administration, reaches the maximum approximately in 1–2 h. The effect lasts up to 24 h. Level of concentration of Timololum in the 1st and in the 2nd hour after introduction of 2 drops of Timololum of 0.5% is 150 ng/100 mg. For 7 h the level of concentration decreased to 10 ng/100 mg.
Radioactivity equivalent 1–10 ng Timololum / 100 fabric mg, was recorded in a cornea, the third century, an iris of the eye/ciliary body.
System absorption: researches showed that Timololum is absorbed after topical administration in an eye. Researches showed that Timololum was revealed in urea of all healthy volunteers and patients. (Timololum the maleate and its metabolites are excreted mainly by kidneys).
Level in blood: concentration of Timololum in blood plasma after topical administration in the recommended clinical doses cannot often be defined (less than 2 ng/ml), and neither after single introduction of a dose, nor after 2 weeks treatment.
With max in blood plasma after application of 2 drops 2 times a day made 9.6 ng/ml. It was noted in 30–90 min. after application.
Indication
Increased intraocular pressure (eye hypertensia); glaucoma (chronic open angle glaucoma); glaucoma after removal of lenses (glaucoma at an aphakia).
Use
to Dig in on 1 drop arutimol 0.25% of solution in the affected eye (eyes) 2 times a day. if result unsatisfactory, it is necessary to apply 0.5% solution.
If intraocular pressure at regular application is established byat the desirable level, a dose it is possible to lower to 1 drop 0.25% of solution or, respectively, 0.5% of solution of 1 times a day. When using of contact lenses it is necessary to remove them before Arutimol's application. It is possible to put on them again not earlier than in 15 min.
Bottle is vozdukho- and waterproof. From above it is leaky closed by a cap. Before the first use it is necessary to close densely a cap, turning it clockwise that the thorn in a cap punctured a bottle dropper. Then it is necessary to turn a cap counterclockwise.
Arutimol, drops eye, dig in in a conjunctival sac of an eye. For this purpose it is necessary to incline the head back, to look up and to delay a lower eyelid from an eye a little. The bottle should be held an opening down. Pressing a bottle, to dig eye drops in a conjunctiva of a lower eyelid. The bottle opening with drops should not contact to an eye. Right after use of medicine it is necessary to perform the following operations: to close eyelids and to press slightly a finger an internal corner of an eye (at a nose) for 1–2 min. to prevent hit of solution in plaintive tubules and, thus, to reduce possible systemic side effect of medicine.
After use should close a bottle. Drops with an open bottle cannot be used longer than during 6 weeks after opening.
As a rule, treatment by Arutimol yields results after quite long period of application. Interruption of a course or change of a dosage are admissible only according to the recommendation of the doctor. In case of the admission of administration of medicament it is necessary to dig the next dose as soon as remembered need of use of medicine. It is not necessary to double the following dose.
Contraindication
Use of an arutimol, a thaw eye, is contraindicated in the presence of the following diseases: bronchial hypersensitivity; oh or oh in the anamnesis; heavy hobl; sinus bradycardia, av-blockade of ii and iii of degree; heart failure; cardiogenic shock.
Arutimol, drops eye, it is also not necessary to appoint at hypersensitivity to any of medicine components, heavy allergic rhinitis or dystrophic violations of a cornea. It is necessary to avoid co-administration of local blockers of β-adrenoceptors, such as Timololum and also orally or in/in medicines — antagonists of calcium the patient with heart failure as deterioration in AV conductivity, left-side heart failure and arterial hypotension is possible.
Drop eye is not recommended to apply Timololum to treatment of patients at whom increase in eye pressure is noted at night.
Side effects
are possibleAt use of Timololum the following side reactions:
Disturbance of the general character and a state, connected with the injection site: headache, adynamy, stethalgia.
from a cardiovascular system: bradycardia, arrhythmia, arterial hypotension, syncope, warm blockade, stroke, ischemia of vessels of a brain, stagnant heart failure, tachycardia, cardiac arrest.
from digestive system: diarrhea, nausea.
from nervous system: dizziness, strengthening of objective and subjective symptomatology of a myasthenia of gravis, paresthesia.
Mental violations: depression.
from skin and hypodermic fabrics: hypersensitivity, including the generalized and localized rash, a small tortoiseshell, an alopecia.
from airways, thoracic and mediastinal violations: a bronchospasm (especially at patients with earlier existing diseases of lungs), respiratory insufficiency, short wind, congestion of a nose, cough.
Endocrine violations: the larvate symptoms of a hypoglycemia at patients with insulin-dependent diabetes mellitus.
Ophthalmologic violations: objective and subjective symptomatology of irritation of eyes, including conjunctivitis, blepharitis, a keratitis, a blepharoptosis, decrease in sensitivity of a cornea, a disorder of vision, including changes of a refraction (in certain cases because of the termination of therapy using miotocs), xerophthalmus, a diplopia, a ptosis.
bygave Below side reactions about which messages arrived, but the causal interrelation with use of Timololum was not established.
Disturbance of the general character and a state, connected with the injection site: increased fatigue.
from a cardiovascular system: AG, lung hypostasis, stenocardia.
from digestive system: dyspepsia, anorexia, dryness in a mouth.
Disturbance from nervous system / mental violations: behavior disorders, including confusion, hallucinations, alarm, a disorientation, excitement, drowsiness and other mental disorders.
Ophthalmologic violations: tsistoidny macular hypostasis.
from an urinary system: retroperitoneal fibrosis, impotence.
Following side reactions were recorded byin clinical practice at oral administration of Timololum of a maleate, and can arise at ophthalmologic use of Timololum of a maleate.
Disturbance of the general character and a state, connected with the injection site: extremity pain, decrease in level of physical activities, degrowth of a body.
from a cardiovascular system: hypostasis, deterioration in a course of arterial insufficiency, Raynaud's disease, vazodilatation.
from digestive system: pain in a stomach or intestines, a hepatomegalia, vomiting.
from blood and lymphatic system: purple.
Endocrine violations: hyperglycemia, hypoglycemia.
from skin and hypodermic fabrics: an itch, irritation of skin, the increased pigmentation, sweating, a pokholodeniye of extremities.
from a musculoskeletal system and connective tissue: arthralgia, lameness.
from nervous system / mental violations: dizziness, weakness, decrease in sexual desire, nightmares, insomnia, decrease in concentration of attention.
from airways, thoracic and mediastinal violations: rattles, bronchial obstruction.
from an organ of hearing: sonitus.
from kidneys and urinary tract: the complicated urination.
Possible side reactionsFollowing side reactions were recorded by
at application of other blockers of β-adrenoceptors and can arise at ophthalmologic use of Timololum of a maleate.
from digestive system: mezenterialny arterial thrombosis, ischemic colitis.
from blood and lymphatic system: agranulocytosis, Werlhof's disease.
from nervous system: a reverse depression which progresses in a catatonia; the sharp reverse syndrome, is characterized by a disorientation in time and space, short-term loss of memory, emotional lability, insignificant turbidity of consciousness and reduced indicators of neuropsychometrics.
from the immune system: erythematic rash, fever which is followed by pain and inflammation of a throat, a laryngospasm with respiratory insufficiency.
from an urinary system: Peyroni's disease.
messages about emergence of the syndrome including psoriazopodobny rash on skin, the dry conjunctivitis, otitis and a sclerosing serositis connected with application of blockers of β-adrenoceptors, Practololum Arrived. About emergence of this syndrome at use of Timololum of a maleate it was not reported.
Special instructionsBlockers of β-adrenoceptors applied locally can be absorbed by
systemically. thus, at topical administration of medicine the same side reactions are possible, as well as at system application of blockers of β-adrenoceptors. for example, after use of Timololum it was reported about serious reactions from respiratory and cardiovascular systems including about lethal cases as a result of a bronchospasm at patients with oh and seldom in connection with heart failure. by
received the message that blockers of β-adrenoceptors can cause weakness of muscles that is connected with certain symptoms of a myasthenia (for example a diplopia, a ptosis and the general weakness). Also sometimes messages arrived that Timololum causes weakness of muscles in some patients with a myasthenia of gravis or with myasthenia symptoms.
Main problem of treatment at patients with closed-angle glaucoma is need of opening of a corner. For this purpose it is necessary to narrow a pupil by means of miotocs, Timololum practically does not influence a pupil.
If Timololum is applied byto decrease in the increased intraocular pressure at closed-angle glaucoma, it should be applied together with miotika. As well as at use of other protivoglaukomny medicines, at some patients noted decrease in sensitivity to Timololum after long therapy.
Before the general anesthesia should stop gradually application of blockers of β-adrenoceptors as they reduce ability of heart to react to stimulation of β-adrenoceptors of a sympathetic system.
forIf in addition to apply other eye means, it is necessary to wait 15 min. between their application.
Risk of emergence of anaphylactic reactions: patients with an atopy or with heavy pathological reactions to numerous allergens in the anamnesis can react more sharply to repeated accidental diagnostic or therapeutic doses of these allergens if they accepted blockers of β-adrenoceptors. Such patients can not react to a usual dose of adrenaline which is appointed in case of anaphylactic reactions.
during treatment by means of the medicine Arutimol should take regularly measurement of intraocular pressure and inspection of a cornea of an eye.
After operational treatment of glaucoma and at use of the medicines reducing secretion of intraocular moisture were noted by cases of peeling of a choroid of an eye. Such cases were described at use of Timololum and acetazoleamide.
Because of potential impact of blockers of β-adrenoceptors on the ABP and ChSS these means should be applied with care at patients with insufficiency of cerebral circulation. If after the beginning of therapy using Timololum signs or symptoms of decrease in cerebral circulation develop, it is necessary to perform an alternative type of therapy.
byAt use of the medicine Arutimol, a drop eye, the positive take at doping control can be revealed.
Benzalkoniya'schloride can cause irritation of an eye. Therefore it is necessary to avoid contact of medicine with soft contact lenses. Benzalkoniya can lead chloride to discoloration of soft contact lenses. Contact lenses need to be removed before burying and to put on again not earlier than in 15 min. It should be noted that at stabilization of intraocular pressure its initial decrease can be up to 50% then the efficiency of medicine can decrease (tachyphylaxis). During the period from the 3rd to the 12th month the pressure decrease is stabilized. Therefore it is important to exercise regular control of pressure during the first 5 days from the moment of application of eye drops of Timololum.
At application of blockers of β-adrenoceptors the decrease in intraocular pressure is orally possible. In that case it is necessary to consider further need of topical administration of eye drops of Timololum. If blockers of β-adrenoceptors were already applied systemically, the additional effect of medicines for topical administration is usually lower. It is necessary to carry out regular monitoring of the patients who are in addition applying blockers of β-adrenoceptors orally or for which application of blockers of β-adrenoceptors is limited.
At patients should not apply two local ophthalmologic blockers of β-adrenoceptors at the same time.
At patients with much the pigmented iris of the eye pressure decrease can be noted with delay and in weaker degree.
After the treatment termination the effect of medicine can proceed within several days. If treatment by Timololum, eye drops, is stopped after prolonged use, its action for decrease in intraocular pressure can last 2–4 weeks
At instillation only in one eye the blockers of β-adrenoceptors can have effect on decrease in intraocular pressure and on an uncured eye.
Period of pregnancy and feeding by a breast. Any data of clinical trials on use of medicine for pregnant women are absent. Arutimol, drops eye, it is not necessary to apply during pregnancy and feeding by a breast.
Use of medicine before childbirth can give, as well as with other blockers of β-adrenoceptors, to bradycardia, a hypoglycemia and respiratory depression (neonatal asphyxia) at the newborn. For other blockers of β-adrenoceptors cases of β-blockade were described. For this reason it is necessary to carry out continuous monitoring of a condition of newborns during the first several days of their life.
After instillation in an eye Timololum gets into breast milk where it can accumulate in higher concentrations, than in blood plasma. Though the amount of active ingredient which thus is reached in breast milk probably does not represent risk for babies, and identification of symptoms of β-blockade requires high-quality inspection.
Children. The efficiency and safety of use of medicine for children are not established.
Ability to influence speed of response at control of vehicles or work with mechanisms. Even at appropriate application the medicine Arutimol can affect sight and also worsen speed of response in such degree that it can affect ability to steer vehicles or to work with mechanisms.
At simultaneous application of the eye drops containing adrenaline the mydriasis is possible
Interaction. the combined application of the eye drops containing adrenaline or pilocarpine strengthens effect of Timololum concerning decrease in intraocular pressure. At simultaneous application of blockers of β-adrenoceptors strengthening as local (decrease in intraocular pressure), and systemic action (influence on a cardiovascular system) is possible
. If eye drops Arutimol apply along with antihypertensive or other cardiological medicaments (for example antagonists of calcium, reserpine, blockers of β-adrenoceptors), risk of developing arterial hypotension and bradycardia increases.
Neuromuscular blockade caused by tubocurarine can be strengthened by action of a blocker of β-adrenoceptors.
Expressiveness of cardiodepressive action can increase in case of simultaneous use of antiarrhythmic medicines with quinidinesimilar action.
Negative chronotropic and dromotropic effect can amplify in case of the combined use of cardiac glycosides.
At simultaneous application of blockers of β-adrenoceptors and β 2 - sympathomimetics the effect of the last can decrease and there can be a bronchospasm.
Combined use of insulin or other anti-diabetic means, especially against the background of a stress or physical activity (hypoglycemia), can cause or increase weight of a latent hypoglycemia.
OverdoseAt observance of the mode of dosing and application the possibility of toxic side effect is almost excluded by
.
Symptoms: the expressed decrease in the ABP, development of heart failure, cardiogenic shock, heavy bradycardia, up to cardiac arrest. Besides, there can be breath dysfunctions, a bronchospasm, violations from a GIT, confusion of consciousness and a spasm.
Treatment: in the conditions of intensive medical care, except the general therapy, it is necessary to carry out monitoring of vital indicators. In case of overdose such antidotes are appointed:
- atropine oxide: 0.5-2 mg in/in;
- glucagon: at first 1–10 mg in/in, then 2-2.5 mg each hour in the form of long infusion;
- β-sympathomimetics depending on the body weight and effect: Dobutaminum, izoprenalin, ortsiprenalin or epinephrine.
In resistant bradycardia should consider treatment by means of a pacemaker. At a bronchospasm can appoint β 2 - sympathomimetics (in the form of aerosol or, if necessary, in / c) or Aminophyllinum in / century. In spasms it is recommended slow in/in diazepam introduction.
Storage conditions
B the place protected from light at a temperature up to 25 °C. after opening of a bottle it is necessary to use medicine for 6 weeks
Specifications
| Characteristics | |
| Active ingredients | Timololum |
| Amount of active ingredient | 5 mg/ml |
| Applicant | Baush Hels |
| Code of automatic telephone exchange | S01ED01 Timololum |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | The branded generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | bottle |
| Producer | OTHER GERHARD OF THE MANNA HAM. - PHARMACEUTICAL. GMBH FACTORIES |
| Quantity in packing | 5 ml |
| Release form | eye drops |
| Route of administration | Eye |
| Sign | Import |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Arutimol |








































